Tuesday September 26 was an exciting day for the PureIMS team. Not only was the first cohort of volunteers dosed in the penultimate Levodopa Cyclops™ bioavailability study, we were also honored with the LIFE Science Innovation Award. LIFE Cooperative, very successfully organizing their annual conference and award ceremony, is a cooperation of 50+ LifeScience and MedTech SMEs […]
PureIMS initiates dose-finding study with Levodopa Cyclops™ dry powder inhaler
Following a successful funding round in April of this year (link: successful funding round), PureIMS obtained ethical approval for a pharmacokinetic dose-finding study with its dry powder inhaler lead program Levodopa Cyclops™ and expects to have the study results before the end of 2023. Earlier clinical studies already demonstrated very rapid onset of action, a […]
PureIMS secures new investment round to develop lead inhalation product and facilitate partnering activities
PureIMS today announced it has secured a new investment round. New investor Boost-UP Foundation has joined current shareholders (CardusoCapital venture capital fund, IMDS Medical Devices and LinesBridge Pharma Group) in this round to finance the company’s ambitious growth strategy. Please find the full press release via this link:
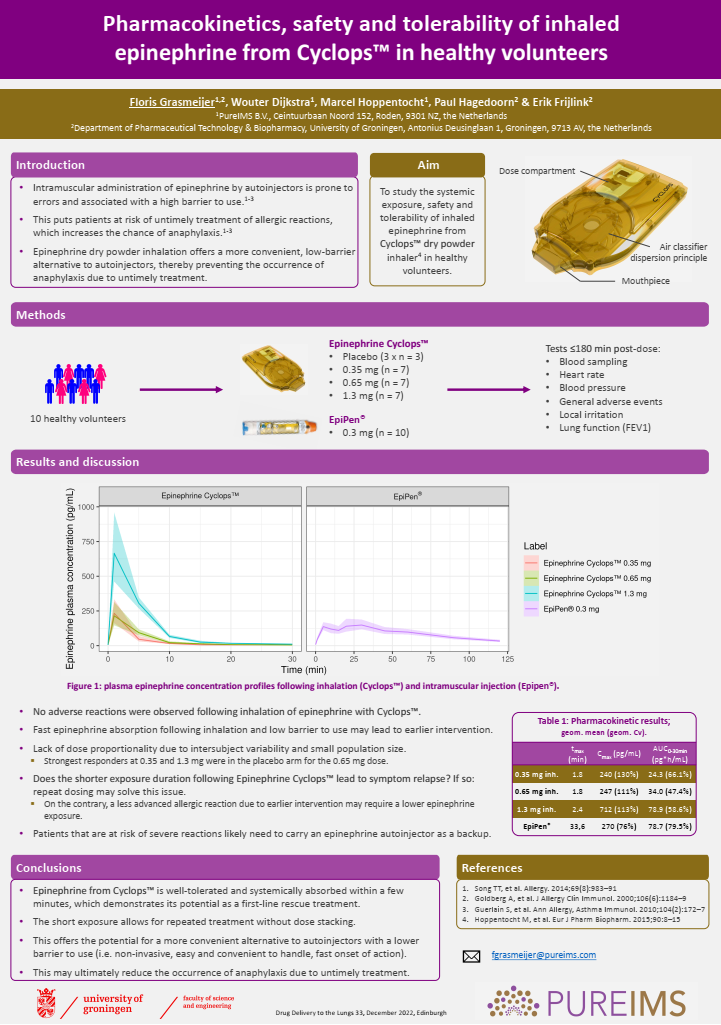
Drug Delivery to the Lungs (DDL) poster on Epinephrine Cyclops™
Check out our scientific poster for DDL2022 on Epinephrine Cyclops™!
PureIMS present at DDL2022
PureIMS will be present at the Drug Delivery to the Lungs Conference 2022 in Edinburgh. Please visit our stand (246) and have a chat. We will be more than happy to tell you everything about our Cyclops™ single-use dry powder inhaler and hear all about your interesting projects too! Furthermore, check out our posters on […]
PureIMS appoints Jaap Wieling, PhD, as new CEO
Roden — January 7, 2022 — PureIMS, a clinical stage pharmaceutical and medication systems company focused on developing and commercializing innovative inhaled therapies for systemic and respiratory diseases has appointed Jaap Wieling to the role of Chief Executive Officer (CEO). Dr. Wieling brings 30 years of experience as an executive and entrepreneur in (bio)pharmaceutical research […]
Levodopa Cyclops™ in Dutch magazine ‘Tijdschrift voor Neurologie en Neurochirurgie (TNN)’
Inhaled levodopa for the rescue treatment of OFF episodes in Parkinson’s disease OFF episodes are an unmet medical need with a great impact on the quality of life of Parkinson’s disease patients. Oral levodopa does not prevent the occurrence of OFF episodes, nor enables their fast resolve, mainly because of a highly variable and unreliable […]
PureIMS participating in Biotech Showcase™ 2022
PureIMS aims to find a partner for its lead program Levodopa Cyclops™ Roden — December 22, 2021 — PureIMS today announced that it is participating in Biotech Showcase™ 2022. PureIMS is a clinical stage pharmaceutical and medication systems company developing a portfolio of products addressing acute and unmet medical needs. These products are based on […]
PureIMS to present at Biotech Showcase™ Digital 2021
PureIMS preparing for Series A financing round aiming to bring its two lead programs to marketing authorization Roden — December 16, 2020 — PureIMS today announced that it is participating in Biotech Showcase™ Digital 2021, and providing an on-demand company presentation. Bram van Dijck, CEO, will be presenting PureIMS at Biotech Showcase Digital. PureIMS is […]
Automated Filling Equipment Allows Increase in the Maximum Dose to Be Filled in the Cyclops® High Dose Dry Powder Inhalation Device While Maintaining Dispersibility
I. Sibum, P. Hagedoorn, C.O. Botterman, H.W. Frijlink and F. Grasmeijer In recent years there has been increasing interest in the pulmonary delivery of high dose dry powder drugs, such as antibiotics. Drugs in this class need to be dosed in doses far over 2.5 mg, and the use of excipients should therefore be minimized. […]