Colistin Cyclops® Gains EMA and FDA Orphan Drug Status in Cystic Fibrosis
EMA as well as FDA recently designated PureIMS’s colistin dry powder inhaler ‘Colistin Cyclops®’ orphan drug product for the treatment of cystic fibrosis. Colistin Cyclops® is an orally inhaled antibiotic used for treating (chronic) Pseudomonas aeruginosa infections of the lungs. Despite the transformative potential of CFTR modulators in addressing the root causes of cystic fibrosis, […]
CYCLOPS® Dry Powder Inhaler Now Available Nationwide in the Netherlands Thanks to Agreement Between Fagron and PureIMS
Fagron and PureIMS have joined forces to make CYCLOPS® dry powder inhaler available nationwide in the Netherlands. The two Dutch pharmaceutical companies recently signed an agreement, granting Fagron the exclusive rights to organize the national distribution of PureIMS’s compounded preparations, such as an inhaled antibiotic for the treatment of pulmonary Pseudomonas aeruginosa infections caused by […]
International Congress of Parkinson’s Disease and Movement Disorders 2024 – poster on Levodopa Cyclops®
Please check out our latest poster (#708) presenting clinical study results of a head-to-head comparison between Levodopa Cyclops® and Inbrija®. Highlights are: Levodopa Cyclops® is safe and very well tolerated (no cough). Levodopa absorption from Cyclops® is comparable to Inbrija®, thereby, fulfilling the bioequivalence criteria. Results enable abbreviated registration routes with a limited – PK-only […]
Cyclops® in Inhalation Technology magazine!
Dry powder inhalers for rescue applications Check out this fascinating article on the potential of Cyclops® for rescue applications, featured in the Inhalation Technology Supplement of the PMPS Spring 2024 edition. Dive into the details through the link below. Don’t miss it!
PureIMS gears up for abbreviated registration of Levodopa Cyclops® against OFF episodes in Parkinson’s disease with the successful completion of a comparative pharmacokinetic study
Roden, the Netherlands, January 18, 2024 PureIMS, a pharmaceutical company focused on the development of innovative inhalation products, this week reported it has completed a comparative pharmacokinetic study with Levodopa Cyclops®. The study results show rapid pulmonary absorption of levodopa in absence of safety and tolerability issues. These properties make Levodopa Cyclops® a prime candidate […]
PureIMS winning the LIFE Science Innovation Award!
Tuesday September 26 was an exciting day for the PureIMS team. Not only was the first cohort of volunteers dosed in the penultimate Levodopa Cyclops™ bioavailability study, we were also honored with the LIFE Science Innovation Award. LIFE Cooperative, very successfully organizing their annual conference and award ceremony, is a cooperation of 50+ LifeScience and MedTech SMEs […]
PureIMS initiates dose-finding study with Levodopa Cyclops™ dry powder inhaler
Following a successful funding round in April of this year (link: successful funding round), PureIMS obtained ethical approval for a pharmacokinetic dose-finding study with its dry powder inhaler lead program Levodopa Cyclops™ and expects to have the study results before the end of 2023. Earlier clinical studies already demonstrated very rapid onset of action, a […]
PureIMS secures new investment round to develop lead inhalation product and facilitate partnering activities
PureIMS today announced it has secured a new investment round. New investor Boost-UP Foundation has joined current shareholders (CardusoCapital venture capital fund, IMDS Medical Devices and LinesBridge Pharma Group) in this round to finance the company’s ambitious growth strategy. Please find the full press release via this link:
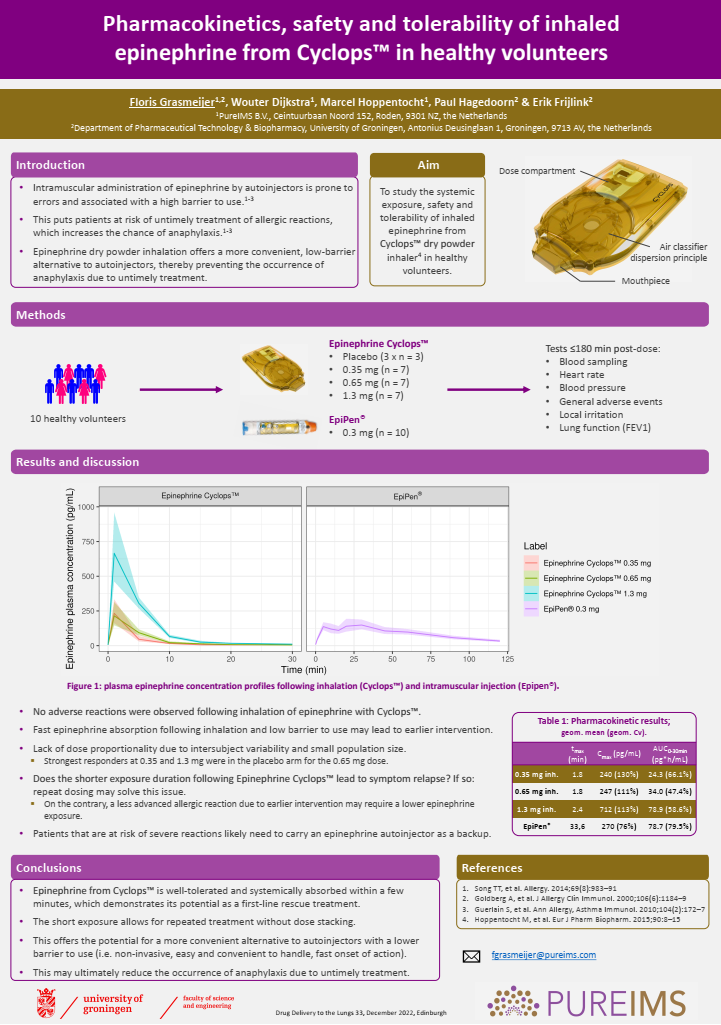
Drug Delivery to the Lungs (DDL) poster on Epinephrine Cyclops™
Check out our scientific poster for DDL2022 on Epinephrine Cyclops™!