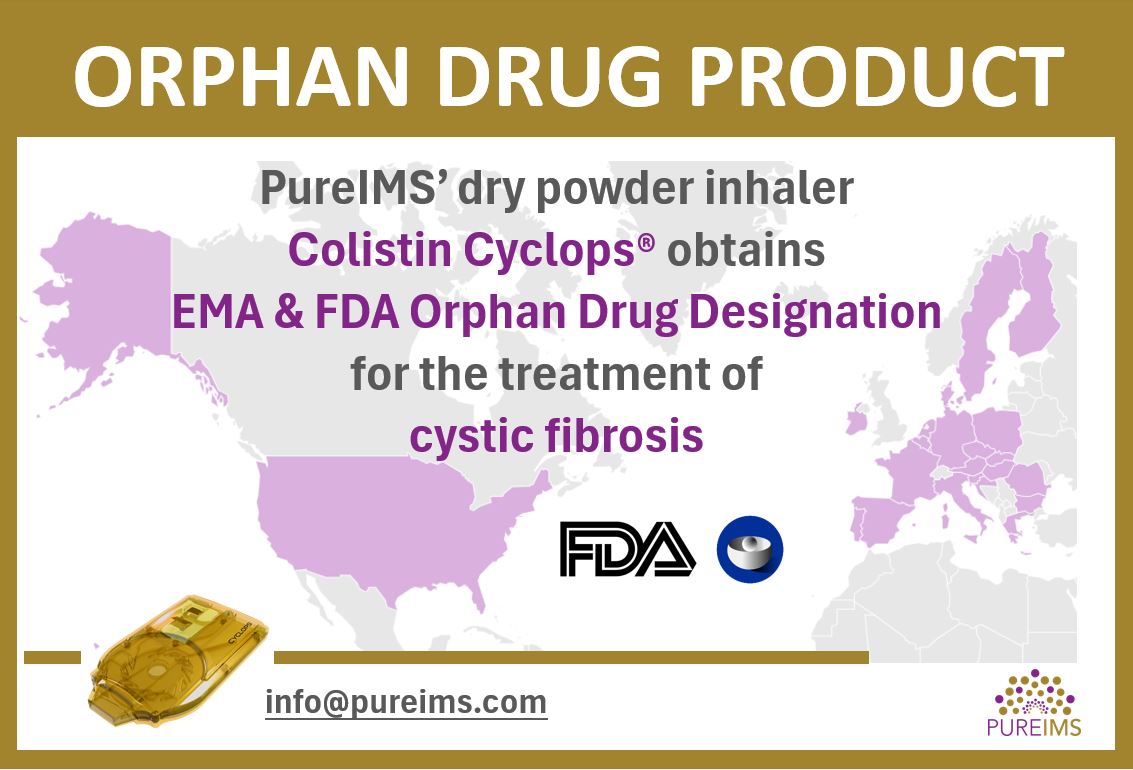
EMA as well as FDA recently designated PureIMS’s colistin dry powder inhaler ‘Colistin Cyclops®’ orphan drug product for the treatment of cystic fibrosis. Colistin Cyclops® is an orally inhaled antibiotic used for treating (chronic) Pseudomonas aeruginosa infections of the lungs. Despite the transformative potential of CFTR modulators in addressing the root causes of cystic fibrosis, […]
Category: Press release
CYCLOPS® Dry Powder Inhaler Now Available Nationwide in the Netherlands Thanks to Agreement Between Fagron and PureIMS
Fagron and PureIMS have joined forces to make CYCLOPS® dry powder inhaler available nationwide in the Netherlands. The two Dutch pharmaceutical companies recently signed an agreement, granting Fagron the exclusive rights to organize the national distribution of PureIMS’s compounded preparations, such as an inhaled antibiotic for the treatment of pulmonary Pseudomonas aeruginosa infections caused by […]
PureIMS gears up for abbreviated registration of Levodopa Cyclops® against OFF episodes in Parkinson’s disease with the successful completion of a comparative pharmacokinetic study
Roden, the Netherlands, January 18, 2024 PureIMS, a pharmaceutical company focused on the development of innovative inhalation products, this week reported it has completed a comparative pharmacokinetic study with Levodopa Cyclops®. The study results show rapid pulmonary absorption of levodopa in absence of safety and tolerability issues. These properties make Levodopa Cyclops® a prime candidate […]
PureIMS initiates dose-finding study with Levodopa Cyclops™ dry powder inhaler
Following a successful funding round in April of this year (link: successful funding round), PureIMS obtained ethical approval for a pharmacokinetic dose-finding study with its dry powder inhaler lead program Levodopa Cyclops™ and expects to have the study results before the end of 2023. Earlier clinical studies already demonstrated very rapid onset of action, a […]
PureIMS secures new investment round to develop lead inhalation product and facilitate partnering activities
PureIMS today announced it has secured a new investment round. New investor Boost-UP Foundation has joined current shareholders (CardusoCapital venture capital fund, IMDS Medical Devices and LinesBridge Pharma Group) in this round to finance the company’s ambitious growth strategy. Please find the full press release via this link:
PureIMS appoints Jaap Wieling, PhD, as new CEO
Roden — January 7, 2022 — PureIMS, a clinical stage pharmaceutical and medication systems company focused on developing and commercializing innovative inhaled therapies for systemic and respiratory diseases has appointed Jaap Wieling to the role of Chief Executive Officer (CEO). Dr. Wieling brings 30 years of experience as an executive and entrepreneur in (bio)pharmaceutical research […]
PureIMS participating in Biotech Showcase™ 2022
PureIMS aims to find a partner for its lead program Levodopa Cyclops™ Roden — December 22, 2021 — PureIMS today announced that it is participating in Biotech Showcase™ 2022. PureIMS is a clinical stage pharmaceutical and medication systems company developing a portfolio of products addressing acute and unmet medical needs. These products are based on […]
PureIMS to present at Biotech Showcase™ Digital 2021
PureIMS preparing for Series A financing round aiming to bring its two lead programs to marketing authorization Roden — December 16, 2020 — PureIMS today announced that it is participating in Biotech Showcase™ Digital 2021, and providing an on-demand company presentation. Bram van Dijck, CEO, will be presenting PureIMS at Biotech Showcase Digital. PureIMS is […]
PureIMS Initiates Phase 1 Clinical Trial to Evaluate Dry Powder Inhaler Formulation of Amikacin for Early Eradication Treatment of Tuberculosis
Delivery via inhaler could dramatically expand Amikacin’s clinical utility by immediate reduction of the contagiousness of Mycobacterium tuberculosis. Roden — July 1st, 2020 — PureIMS, a clinical stage biopharmaceutical company focused on developing and commercializing innovative therapies for respiratory and systemic diseases, announces today the initiation of a Phase 1 clinical trial to evaluate the […]
Hydroxychloroquine Cyclops in Dutch pharmacy magazine ‘Pharmaceutisch Weekblad’
In the fight against COVID-19, PIMS Hydroxychloroquine (subsidiary of PureIMS) – together with (among others) the University of Groningen, UMCG and IMDS – is working feverishly on inhaled hydroxychloroquine. Following pulmonary administration, we expect a much greater effect of the drug with a lower chance of side effects, because it is administered directly to the […]