Centered around our proprietary inhaler Cyclops®, PureIMS provides a range of compelling business opportunities tailored for biotech and pharmaceutical companies:
- In-house pipeline programs are available for in-licensing: Explore our diverse pipeline of innovative products available for in-licensing, offering a strategic pathway to enrich your portfolio and accelerate your development efforts.
- PureIMS supporting partners with a Cyclops®-based inhalation route for their API/formulation of choice: Collaborate with us to leverage the Cyclops® inhaler platform for delivering your dry powder formulations, empowering you to enhance the efficacy and accessibility of your therapeutic offerings.
Below, we showcase our in-house pipeline products. For further details on our co-development initiatives, please refer to our dedicated section, or click here.
In-house pipeline programs
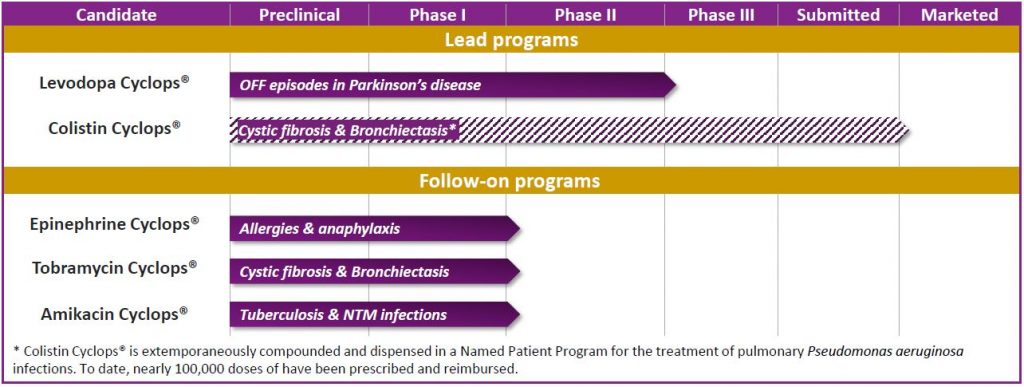
Lead programs
Levodopa Cyclops®
Levodopa Cyclops® is a levodopa dry powder inhaler for the fast and reliable relief of OFF episodes in Parkinson’s disease. As such, Levodopa Cyclops® answers a major unmet medical need.
Levodopa is the cornerstone in the treatment of Parkinson’s disease for well over 50 years. It is the most effective, safe and well tolerated drug available to this end. However, patients suffering from Parkinson’s disease invariably will encounter a major unmet medical need as the disease progresses:
- Patients in mid- and late-stage disease suffer from variable therapeutic efficacy, which results in periodic motor-fluctuations (ON and OFF episodes).
- Patients with acute OFF episodes need fast and reliable rescue therapy to be able to participate in daily activities.
- Currently available medication either has a slow or unpredictable onset of action or is poorly tolerated and uncomfortable or cumbersome to use.
Inhaled levodopa from the Cyclops® offers Parkinson’s disease patients easier and more convenient handling, a faster onset of action and more flexible dosing than most other treatments to counteract OFF episodes.
Studies showed that Parkinson’s disease patients are able to readily use Levodopa Cyclops® during an OFF episode. Inhaled levodopa from the Cyclops® results in therapeutic plasma concentrations within minutes and its absorption is less variable than that of oral levodopa, both of which are prerequisites for the rapid and predictable relief of OFF episodes.
Colistin Cyclops®
Colistin Cyclops® is a pre-filled colistin dry powder inhaler for the treatment of pulmonary Pseudomonas aeruginosa infections in cystic fibrosis and bronchiectasis. It offers a more patient-friendly alternative to other methods of administering colistin, because it is easy to use, easy to carry, pre-filled and disposable (no cleaning required!).
Inhaled colistin is a safe and effective treatment for (chronic) infections of the lungs with Pseudomonas aeruginosa. To date, inhalation of colistin by nebulization has been a very time consuming endeavour, whereas colistin dry powder inhalation from capsule based inhalers often results in pronounced cough. Colistin Cyclops® offers a fast and convenient alternative to colistin inhalation.
Since 2015 Colistin Cyclops® (and its precursor Colistin Twincer™) are available in the Netherlands for named patient use. Read more information about our Colistin Cyclops® named patient use and the experience of patients and health care providers with Colistin Cyclops® by clicking the link.
Follow-on programs
Epinephrine Cyclops®
Epinephrine Cyclops® is a pre-loaded disposable breath-powered epinephrine dry powder inhaler for the fast and reliable treatment of severe allergic reactions and impending anaphylaxis. Inhaled epinephrine from Cyclops® offers persons suffering from food allergy and impending anaphylaxis a low barrier to use because it is non-invasive, easy and convenient to handle, has a fast onset of action and a short exposure that allows for repeated treatment without dose stacking. Epinephrine Cyclops® may also provide direct and effective local treatment of symptoms in the airways and oropharyngeal region. This may especially prove to be beneficial in food allergy patients with asthma.
Epinephrine Cyclops® is more affordable than epinephrine auto-injectors and its credit card size enables excellent portability of one or more devices. This allows patients to have multiple devices per user. It also allows for the disposable nature of Epinephrine Cyclops®. Disposable inhalers are ideal for incidental acute treatment of anaphylaxis.
The current first-aid treatment to prevent anaphylaxis is intramuscular epinephrine injection by means of an autoinjector. Major unmet medical needs experienced by people at risk of anaphylaxis are the following:
- People are reluctant to use their autoinjector, which puts them at risk of untimely treatment of an anaphylactic reaction.
- Fewer than 50% of people at risk of anaphylaxis can use their autoinjector device correctly. An easier-to-use device for epinephrine administration is therefore required.
Epinephrine Cyclops® answers both medical needs with its ease and convenience of use. Besides needle phobia among patients, there are several more reasons to choose a needle-free epinephrine administration:
- Discrete administration method: no strange looks from bystanders when used in public.
- No risk of stick or laceration injuries.
- No accidental injections.
- No risk of erroneous injection in body fat instead of muscle tissue.
- No spare auto-injector available after incorrect use or insufficient effect.
In addition, injecting a loved-one – when he or she is unable to use an auto-injector (like younger children) – may also be traumatic.
A clinical study showed that Epinephrine Cyclops® results in a shorter and less variable time to peak plasma concentration than intramuscular epinephrine. The exposure duration following the use of Epinephrine Cyclops® is also very brief and consistent, which results in a plasma profile that approaches intravenous epinephrine.
Tobramycine Cyclops®
Pulmonary Pseudomonas aeruginosa infections may be treated by continuous alternating therapy with inhaled colistin and inhaled tobramycin. Therefore, in addition to Colistin Cyclops®, PureIMS also develops Tobramycin Cyclops®, a tobramycin dry powder inhaler. This offers patients the ease and comfort of use of the Cyclops® throughout their continuously alternating treatment cycle.
Amikacin Cyclops®
Amikacin Cyclops® is an amikacin dry powder inhaler for the treatment of tuberculosis (TB) or non-tuberculous mycobacterial (NTM) infections. Inhaled amikacin from the Cyclops® will result in higher amikacin concentrations in the lungs than conventional infusion or intramuscular injection. This could more rapidly decrease contagiousness by sterilizing the upper airways of patients with ’open’ (smear positive) TB, potentially even of otherwise drug resistant TB. In addition, Amikacin Cyclops® offers TB patients an easier and much more convenient administration than infusion or injection. A fast reduction of contagiousness and new, less toxic drug regimens with a shorter duration will prevent long hospital admissions and thereby help to reduce healthcare costs.
Amikacin is an approved drug for the treatment of and non-tuberculous mycobacterial infections. Amikacin is not absorbed via the gastrointestinal tract, which makes its oral administration impossible. TB is the world’s most common infectious disease that caused a staggering 1.4 million deaths in 2019 (WHO). Therefore, stopping the spread of TB and improving its treatment are urgently required. Inhaled, more targeted treatment with inhaled amikacin from the Cyclops® may aid in battling the often unrecognized humanitarian disaster TB causes on a global scale.
An open-label Phase 1 clinical trial with Amikacin Cyclops® in TB patients – to determine its pharmacokinetics and local tolerability – was recently completed.






