Cyclops®
Pre-filled
dry Powder inhaler

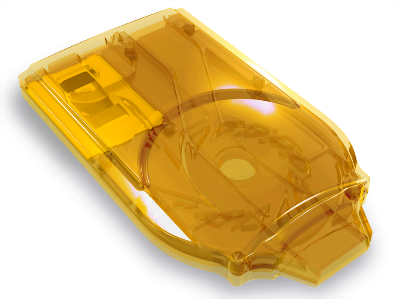
Cyclops® is a state-of-the-art, easy to use, pre-filled DPI that is developed by PureIMS for inhalation powders.
Because of its simple yet
sophisticated proprietary design it can be produced in a cost-effective way.
Cyclops® is available in single & multi use versions.
Upon inhalation it uses the patient’s breath to disperse the dry powder formulation into small particles appropriately sized for deep lung deposition. Cyclops® has several advantages compared to standard-of-care DPIs across key therapeutic areas. These attributes enable the hygienic and effective use on a worldwide scale. One product, Colistin Cyclops®, is already available to patients and reimbursed under a named patient regimen for the treatment of cystic fibrosis.
HIGHLY PREFERRED BY PATIENTS
LATEST NEWS
Head of CMC (Chemistry, Manufacturing, and Controls)
Leads strategy and execution for drug substance/product development, manufacturing (internal
December 19, 2025

PureIMS secures a European grant to develop a new dry powder inhaler
PureIMS receives a grant of € 41.028,58 to initiate
September 3, 2025

An In Vitro–In Vivo Comparison of Two Levodopa Dry Powder Products for Inhalation: A Randomized Trial Comparing Inbrija and Levodopa Cyclops
Full paper: Pharmaceutics 2025, 17(9), 1149; https://doi.org/10.3390/pharmaceutics17091149 Background/Objectives: The pulmonary
September 2, 2025
Jaap Wieling will present Epinephrine Cyclops® on GAFA
Please check out our latest poster on Epinephrine Cyclops® for
August 27, 2025