PureIMS is per direct op zoek naar een productie-apotheker. Als productie-apotheker ben je verantwoordelijk voor de farmaceutische ontwikkeling, productie en kwaliteitsborging van onze droogpoederinhalatoren. Dit omvat zowel de reguliere, groeiende productie van een magistrale bereiding, als de incidentele bereiding van studiemedicatie (IMP’s) ten behoeve van verdere ontwikkeling van onze producten. Je overziet het productieproces van […]
Farmaceutisch Productiemedewerker
PureIMS is een gespecialiseerd farmaceutisch bedrijf dat zich toelegt op de ontwikkeling en commercialisering van geneesmiddelen geschikt voor inhalatie. Om onze productie te ondersteunen, zijn wij op korte termijn op zoek naar een: Farmaceutisch Productiemedewerker Functieomschrijving: Als farmaceutisch productiemedewerker werk je binnen het productieteam en ondersteun je de productie van geneesmiddelen. Je werkt volgens vaste […]
Head of CMC (Chemistry, Manufacturing, and Controls)
Leads strategy and execution for drug substance/product development, manufacturing (internal and external), supply chain and CMC-sections of regulatory filings, from early-clinical development through commercialization, ensuring quality and GMP compliance while managing teams, timelines, and external partners for reliable supply of R&D, clinical and commercial materials. Key Responsibilities: Strategy & Leadership: Develop and implement overall […]
PureIMS secures a European grant to develop a new dry powder inhaler
PureIMS receives a grant of € 41.028,58 to initiate the development of a new dry powder inhaler (DPI). This new DPI will contain an innovative dispersion mechanism to enable delivery of medicines such as antibiotics, vaccines, and biologics. Unlike current devices, it will be user-friendly and suitable for vulnerable groups like children and elderly […]
An In Vitro–In Vivo Comparison of Two Levodopa Dry Powder Products for Inhalation: A Randomized Trial Comparing Inbrija and Levodopa Cyclops
Full paper: Pharmaceutics 2025, 17(9), 1149; https://doi.org/10.3390/pharmaceutics17091149 Background/Objectives: The pulmonary administration of levodopa enables a rapid absorption and onset of action, making it a suitable administration route for managing OFF episodes in Parkinson’s disease. Currently, one dry powder product for inhalation (Inbrija) is available on the market, while another (Levodopa Cyclops) is in development. These […]
Jaap Wieling will present Epinephrine Cyclops® on GAFA
Please check out our latest poster on Epinephrine Cyclops® for GAFA 2025! GAFA 2025 is a medical conference on food allergy and anaphylaxis in Padua, Italy from 11–13 September 2025 and Jaap Wieling will attend this conference. Interested? Contact Jaap (jwieling@pureims.com)!
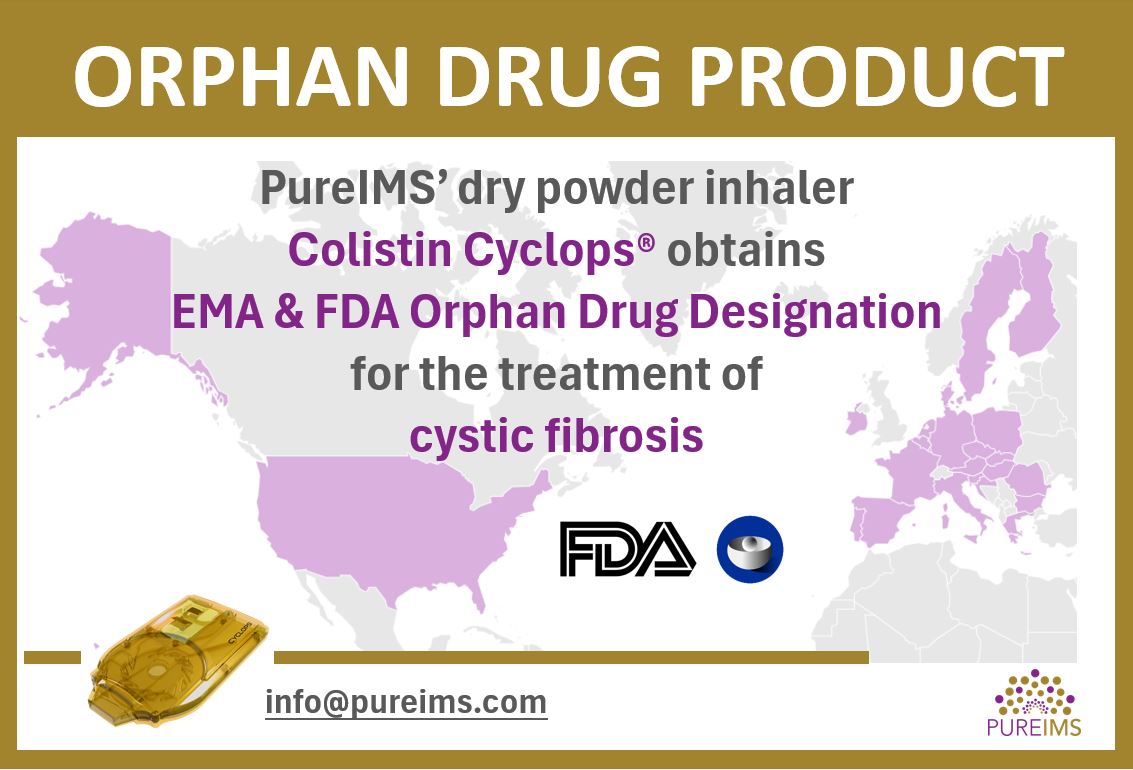
Colistin Cyclops® Gains EMA and FDA Orphan Drug Status in Cystic Fibrosis
EMA as well as FDA recently designated PureIMS’s colistin dry powder inhaler ‘Colistin Cyclops®’ orphan drug product for the treatment of cystic fibrosis. Colistin Cyclops® is an orally inhaled antibiotic used for treating (chronic) Pseudomonas aeruginosa infections of the lungs. Despite the transformative potential of CFTR modulators in addressing the root causes of cystic fibrosis, […]
CYCLOPS® Dry Powder Inhaler Now Available Nationwide in the Netherlands Thanks to Agreement Between Fagron and PureIMS
Fagron and PureIMS have joined forces to make CYCLOPS® dry powder inhaler available nationwide in the Netherlands. The two Dutch pharmaceutical companies recently signed an agreement, granting Fagron the exclusive rights to organize the national distribution of PureIMS’s compounded preparations, such as an inhaled antibiotic for the treatment of pulmonary Pseudomonas aeruginosa infections caused by […]
International Congress of Parkinson’s Disease and Movement Disorders 2024 – poster on Levodopa Cyclops®
Please check out our latest poster (#708) presenting clinical study results of a head-to-head comparison between Levodopa Cyclops® and Inbrija®. Highlights are: Levodopa Cyclops® is safe and very well tolerated (no cough). Levodopa absorption from Cyclops® is comparable to Inbrija®, thereby, fulfilling the bioequivalence criteria. Results enable abbreviated registration routes with a limited – PK-only […]
Cyclops® in Inhalation Technology magazine!
Dry powder inhalers for rescue applications Check out this fascinating article on the potential of Cyclops® for rescue applications, featured in the Inhalation Technology Supplement of the PMPS Spring 2024 edition. Dive into the details through the link below. Don’t miss it!