Full paper: Pharmaceutics 2025, 17(9), 1149; https://doi.org/10.3390/pharmaceutics17091149 Background/Objectives: The pulmonary administration of levodopa enables a rapid absorption and onset of action, making it a suitable administration route for managing OFF episodes in Parkinson’s disease. Currently, one dry powder product for inhalation (Inbrija) is available on the market, while another (Levodopa Cyclops) is in development. These […]
Category: Scientific publication
Jaap Wieling will present Epinephrine Cyclops® on GAFA
Please check out our latest poster on Epinephrine Cyclops® for GAFA 2025! GAFA 2025 is a medical conference on food allergy and anaphylaxis in Padua, Italy from 11–13 September 2025 and Jaap Wieling will attend this conference. Interested? Contact Jaap (jwieling@pureims.com)!
International Congress of Parkinson’s Disease and Movement Disorders 2024 – poster on Levodopa Cyclops®
Please check out our latest poster (#708) presenting clinical study results of a head-to-head comparison between Levodopa Cyclops® and Inbrija®. Highlights are: Levodopa Cyclops® is safe and very well tolerated (no cough). Levodopa absorption from Cyclops® is comparable to Inbrija®, thereby, fulfilling the bioequivalence criteria. Results enable abbreviated registration routes with a limited – PK-only […]
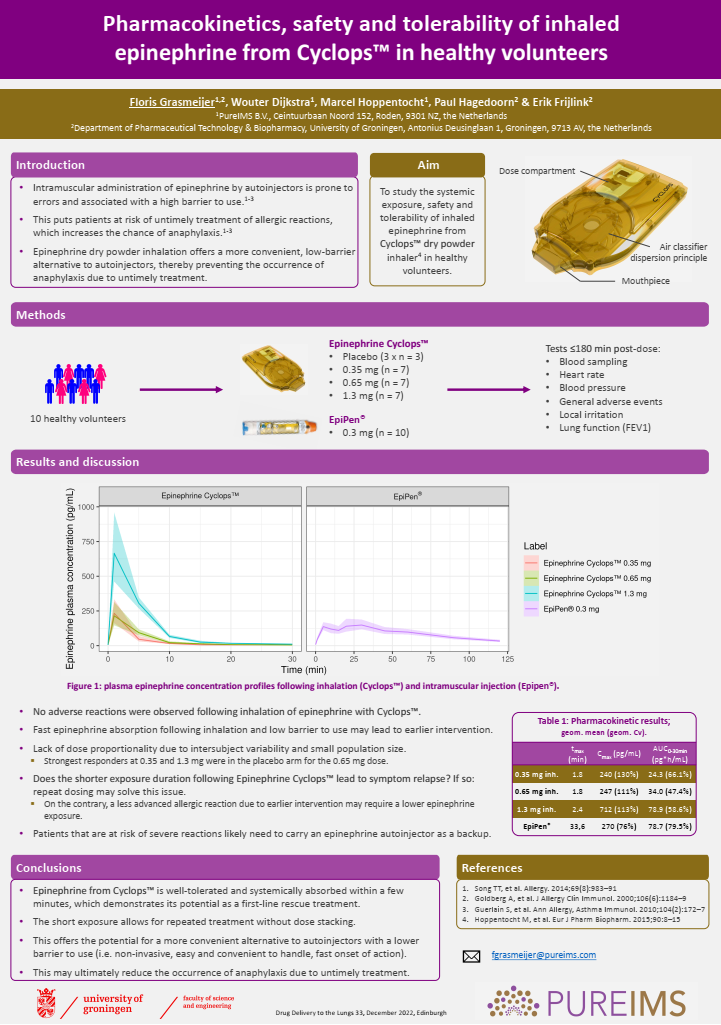
Drug Delivery to the Lungs (DDL) poster on Epinephrine Cyclops™
Check out our scientific poster for DDL2022 on Epinephrine Cyclops™!
Automated Filling Equipment Allows Increase in the Maximum Dose to Be Filled in the Cyclops® High Dose Dry Powder Inhalation Device While Maintaining Dispersibility
I. Sibum, P. Hagedoorn, C.O. Botterman, H.W. Frijlink and F. Grasmeijer In recent years there has been increasing interest in the pulmonary delivery of high dose dry powder drugs, such as antibiotics. Drugs in this class need to be dosed in doses far over 2.5 mg, and the use of excipients should therefore be minimized. […]
Characterization and Formulation of Isoniazid for High-Dose Dry Powder Inhalation
I. Sibum, P. Hagedoorn, H.W. Frijlink, F. Grasmeijer International Journal of Pharmaceutics, 2019, 11 (5) (DOI: 10.3390/pharmaceutics11050233)
Pharmacokinetics and tolerability of inhaled levodopa from a new dry-powder inhaler in patients with Parkinson’s disease
M. Luinstra, A.W.F. Rutgers, T. van Laar, F. Grasmeijer, A. Begeman, V. Isufi, L. Steenhuis, P. Hagedoorn, A.H. de Boer, H.W. Frijlink Pharmacokinetics and tolerability of inhaled levodopa from a new dry-powder inhaler in patients with Parkinson’s disease, Therapeutic Advances in Chronic Disease, 2019, 10 More info
Learning from Parkinson’s patients: Usability of the Cyclops dry powder inhaler
M. Luinstra, V. Isufi, L. de Jong, A.W.F. Rutgers, P. Hagedoorn, J. Puttenstein, T. van Laar, H.W. Frijlink International Journal of Pharmaceutics, 2019, 567, p. 1-5 https://doi.org/10.1016/j.ijpharm.2019.118493