Categories
- Press release (13)
- Publication (3)
- Scientific publication (8)
- Status update (4)
- Vacancies (3)

Productie-apotheker
PureIMS is per direct op zoek naar een productie-apotheker. Als

Farmaceutisch Productiemedewerker
PureIMS is een gespecialiseerd farmaceutisch bedrijf dat zich toelegt op
Head of CMC (Chemistry, Manufacturing, and Controls)
Leads strategy and execution for drug substance/product development, manufacturing (internal

PureIMS secures a European grant to develop a new dry powder inhaler
PureIMS receives a grant of € 41.028,58 to initiate

An In Vitro–In Vivo Comparison of Two Levodopa Dry Powder Products for Inhalation: A Randomized Trial Comparing Inbrija and Levodopa Cyclops
Full paper: Pharmaceutics 2025, 17(9), 1149; https://doi.org/10.3390/pharmaceutics17091149 Background/Objectives: The pulmonary
Jaap Wieling will present Epinephrine Cyclops® on GAFA
Please check out our latest poster on Epinephrine Cyclops® for
Colistin Cyclops® Gains EMA and FDA Orphan Drug Status in Cystic Fibrosis
EMA as well as FDA recently designated PureIMS’s colistin dry

CYCLOPS® Dry Powder Inhaler Now Available Nationwide in the Netherlands Thanks to Agreement Between Fagron and PureIMS
Fagron and PureIMS have joined forces to make CYCLOPS® dry

International Congress of Parkinson’s Disease and Movement Disorders 2024 – poster on Levodopa Cyclops®
Please check out our latest poster (#708) presenting clinical study

Cyclops® in Inhalation Technology magazine!
Dry powder inhalers for rescue applications Check out this fascinating

PureIMS gears up for abbreviated registration of Levodopa Cyclops® against OFF episodes in Parkinson’s disease with the successful completion of a comparative pharmacokinetic study
Roden, the Netherlands, January 18, 2024 PureIMS, a pharmaceutical company

PureIMS winning the LIFE Science Innovation Award!
Tuesday September 26 was an exciting day for the PureIMS team. Not

PureIMS initiates dose-finding study with Levodopa Cyclops™ dry powder inhaler
Following a successful funding round in April of this year
PureIMS secures new investment round to develop lead inhalation product and facilitate partnering activities
PureIMS today announced it has secured a new investment round.
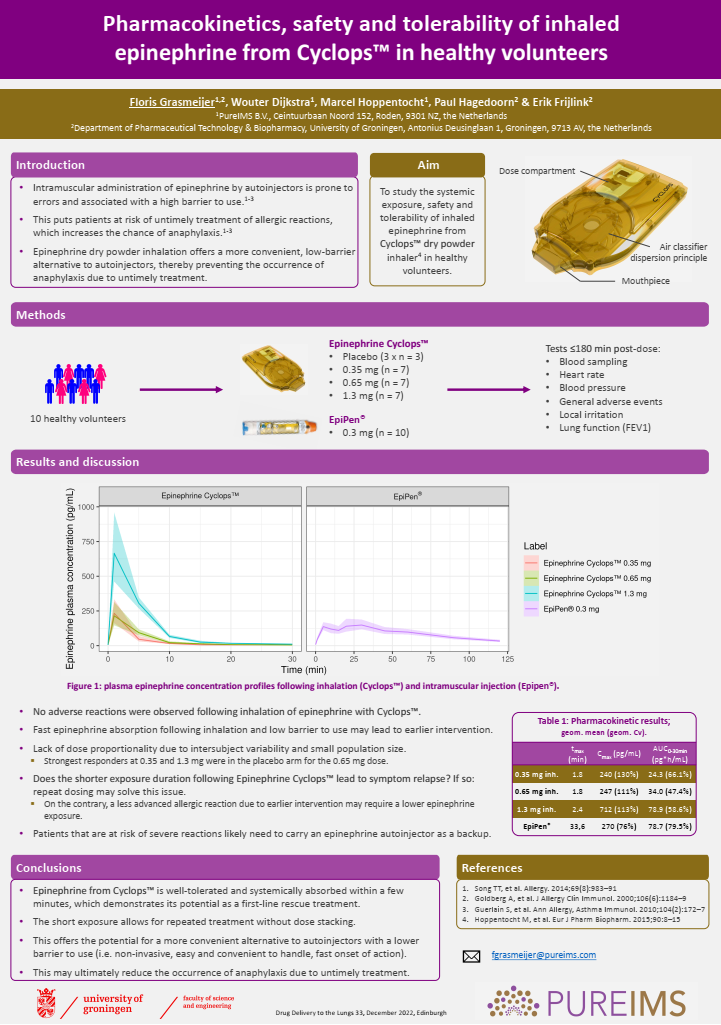
Drug Delivery to the Lungs (DDL) poster on Epinephrine Cyclops™
Check out our scientific poster for DDL2022 on Epinephrine Cyclops™!

PureIMS appoints Jaap Wieling, PhD, as new CEO
Roden — January 7, 2022 — PureIMS, a clinical stage
Levodopa Cyclops™ in Dutch magazine ‘Tijdschrift voor Neurologie en Neurochirurgie (TNN)’
Inhaled levodopa for the rescue treatment of OFF episodes in
PureIMS participating in Biotech Showcase™ 2022
PureIMS aims to find a partner for its lead program
PureIMS to present at Biotech Showcase™ Digital 2021
PureIMS preparing for Series A financing round aiming to bring
Automated Filling Equipment Allows Increase in the Maximum Dose to Be Filled in the Cyclops® High Dose Dry Powder Inhalation Device While Maintaining Dispersibility
I. Sibum, P. Hagedoorn, C.O. Botterman, H.W. Frijlink and F.
PureIMS in Harro Magazine!
We are honoured that Harro Hӧfliger published an article on
PureIMS Initiates Phase 1 Clinical Trial to Evaluate Dry Powder Inhaler Formulation of Amikacin for Early Eradication Treatment of Tuberculosis
Delivery via inhaler could dramatically expand Amikacin’s clinical utility by
Hydroxychloroquine Cyclops in Dutch pharmacy magazine ‘Pharmaceutisch Weekblad’
In the fight against COVID-19, PIMS Hydroxychloroquine (subsidiary of PureIMS)
PureIMS joins the battle against Coronavirus (COVID-19)
In a joint effort with UMCG, University of Groningen and
PureIMS present at DDL
PureIMS will be present at the Drug Delivery to the
Cyclops as example of the TopDutch pioneering and collaborating mentality
The Cyclops™ pre-loaded disposable dry powder inhaler is a product
Carduso Capital invests in PIMS-E
Carduso Capital invests in PIMS-E, a Spin-off company of the
Characterization and Formulation of Isoniazid for High-Dose Dry Powder Inhalation
I. Sibum, P. Hagedoorn, H.W. Frijlink, F. Grasmeijer International Journal
Pharmacokinetics and tolerability of inhaled levodopa from a new dry-powder inhaler in patients with Parkinson’s disease
M. Luinstra, A.W.F. Rutgers, T. van Laar, F. Grasmeijer, A.
Learning from Parkinson’s patients: Usability of the Cyclops dry powder inhaler
M. Luinstra, V. Isufi, L. de Jong, A.W.F. Rutgers, P.


