Cyclops®
Cyclops® is an easy-to-use, pre-filled dpi that is developed for inhalation powders. Upon inhalation it uses the patient’s breath to disperse the dry powder formulation into small particles appropriately sized for deep lung deposition. It can be produced in a cost-effective way because of its simple yet sophisticated proprietary design.
Cyclops® has several advantages compared to standard-of-care DPIs across key therapeutic areas. These attributes enable the hygienic and effective use on a worldwide scale. One product, Colistin Cyclops®, is already available to patients and reimbursed under a named patient regimen for the treatment of cystic fibrosis.
Single- and multi-use versions of Cyclops® available.
Cyclops® High Speed Camera Movie
(Total duration in real life: < 1 second)
- Cyclops® offers therapeutic innovation by way of its unique characteristics and impressive performance:
- High nominal doses ranging from around 5 up to 100 milligrams
- Drug loads of 90-100% due to limited necessity to add excipients to the API
- Very efficient dispersion by its sophisticated classifier leading to fine particle fractions of 75-95%
- A low residue of powder in the inhaler (around 10-20%), deep lung deposition estimated at 50-60% of the nominal dose
Cyclops®Classic
Cyclops®Switch & Cyclops®Charge
In Q3 2024, PureIMS obtained a patent for two different multi-use models, securing protection for a period up to 2043. These new multi-use models increase the flexibility and sustainability of Cyclops® inhaled therapies, while functioning on the basis of the same effective dispersion technique as Cyclops®Classic.
Cyclops®Switch
Cyclops®Charge
Selecting the right Cyclops® model
Selecting the optimal Cyclops® model per therapy will be driven by factors like the required dose, the dosing interval as well as potential medical limitations the patient is facing at the time of taking the drug. During the development stage of the drug-inhaler combination, PureIMS with partners involved will select the best model to offer patients the most effective and user-friendly inhaler type.
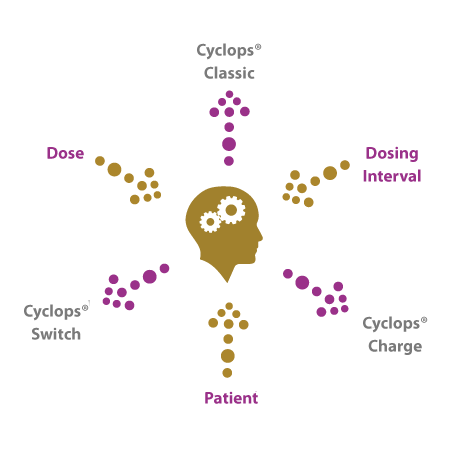
- A few examples:
- For a single dose vaccination program or for an allergy epinephrine rescue therapy, the pre-filled single use Cyclops®Classic will be best suited.
- Parkinson’s Disease patients in a so called ‘off period’ suffering limited motor control have good reasons to use the Cyclops®Classic as well.
-
For therapies where patients will have a choice of titrating the dose, depending on the severity of symptoms, treating physicians might prefer prescribing the Cyclops®Switch.
- A combination therapy with two medications to be dosed in separate inhalations will also be best supported by the Cyclops®Switch.
- When the formulation allows for seven daily dosages, the Cyclops®Charge can be used for a week, without the need for cleaning.
Literature
- J.M.E. Berends, E.J. Wimmenhove, M. Hoppentocht, P. Hagedoorn, H.W. Frijlink, F. Grasmeijer An In Vitro–In Vivo Comparison of Two Levodopa Dry Powder Products for Inhalation: A Randomized Trial Comparing Inbrija and Levodopa Cyclops, Pharmaceutics, 2025;17(9) (DOI: https://doi.org/10.3390/pharmaceutics17091149)
- L. de Jong, M. Luinstra, A.F. Aalbers, A.J. Wijma-Vos, E. D'Angremont, A.A.E. van der Meulen, A.W.F. Rutgers, L. Steenhuis, P. Hagedoorn, T. van Laar, H.W. Frijlink Therapeutic effect of an inhaled levodopa dry powder formulation on off episodes in patients with Parkinson’s disease,Therapeutic Advances in Neurological Disorders, 2024;17 (DOI: https://doi.org/10.1177/17562864241289207)
- R. Heida, R. Akkerman, P.H. Jacob Silva, A.J. Lakerveld, D. Ortiz, C. Bigogno, M. Gasbarri, P.B. van Kasteren, F. Stellacci, H.W. Frijlink, A.L.W. Huckriede, W.L.J. Hinrichs Development of an inhalable antiviral powder formulation against respiratory syncytial virus, Journal of Controlled Release, Volume 357, 2023, (DOI: https://doi.org/10.1016/j.jconrel.2023.03.059))
- I. Sibum, P. Hagedoorn, C.O. Botterman, H.W. Frijlink, F. Grasmeijer, Automated filling equipment allows increase in the maximum dose to be filled in the Cyclops™ high dry powder inhalation device while maintaining dispersibility, International Journal of Pharmaceutics, 2020, 12 (7), 645 (DOI: https://doi.org/10.3390/pharmaceutics12070645)
- I. Sibum, P. Hagedoorn, M. Kluitman, H.W. Frijlink, F. Grasmeijer, Dispersibility and storage stability optimization of high dose isoniazid dry powder inhalation formulations with L-leucine or trileucine, International Journal of Pharmaceutics, 2020, 12 (1) (DOI: 10.3390/pharmaceutics12010024)
- A.M. Akkerman-Nijland, F. Grasmeijer, H.A.M. Kerstjens, H.W. Frijlink, H. van der Vaart, J.M. Vonk, et al., Colistin dry powder inhalation with the Twincer™: An effective and more patient friendly alternative to nebulization, PLoS ONE, 2020, 15 (9): e0239658 (DOI: https://doi.org/10.1371/journal.pone.0239658)
- M. Luinstra, A.W.F. Rutgers, T. van Laar, F. Grasmeijer, A. Begeman, V. Isufi, L. Steenhuis, P. Hagedoorn, A.H. de Boer, H.W. Frijlink, Pharmacokinetics and tolerability of inhaled levodopa from a new dry-powder inhaler in patients with Parkinson's disease, Therapeutic Advances in Chronic Disease, 2019, 10 (DOI: 10.1177/2040622319857617)
- M. Luinstra, V. Isufi, L. de Jong, A.W.F. Rutgers, P. Hagedoorn, J. Puttenstein, T. van Laar, H.W. Frijlink, Learning from Parkinson’s patients: Usability of the Cyclops dry powder inhaler, International Journal of Pharmaceutics, 2019, 567, p. 1-5 (DOI: https://doi.org/10.1016/j.ijpharm.2019.118493)
- M. Hoppentocht, O.W. Akkerman, P. Hagedoorn, J-W.C. Alffenaar, T.S. van der Werf, H.A.M. Kerstjens, H.W. Frijlink, A.H. de Boer, Tolerability and Pharmacokinetic Evaluation of Inhaled Dry Powder Tobramycin Free Base in Non-Cystic Fibrosis Bronchiectasis Patients, 2016, PloS ONE, 11 (3), e0149768 (DOI: https://doi.org/10.1371/journal.pone.0149768)
- M. Hoppentocht, O.W. Akkerman, P. Hagedoorn, H.W. Frijlink, A.H. de Boer, The Cyclops for pulmonary delivery of aminoglycosides; a new member of the Twincer family. European Journal of Pharmaceutics and Biopharmaceutics, 2015, (DOI: 10.1016/j.ejpb.2015.01.012)
- M. Luinstra, A.W.F. Rutgers, H. Dijkstra, F. Grasmeijer, P. Hagedoorn, J.M.J. Vogelzang, et al., Can Patients with Parkinson’s Disease Use Dry Powder Inhalers during Off Periods?, 2015, PLoS ONE 10 (7), e0132714 (DOI: https://doi.org/10.1371/journal.pone.0132714)
- M. Luinstra, F. Grasmeijer, P. Hagedoorn, J.R. Moes, H.W. Frijlink, A.H. de Boer, A levodopa dry powder inhaler for the treatment of Parkinson's disease patients in off periods, European Journal of Pharmaceutics and Biopharmaceutics, 2015, 97 (part A), p. 22-29 (DOI: https://doi.org/10.1016/j.ejpb.2015.10.003)
- A.H. de Boer, P. Hagedoorn, R. Woolhouse, E. Wynn, Computational fluid dynamics (CFD) assisted performance evaluation of the Twincer™ disposable high-dose dry powder inhaler, Journal of Pharmacy and Pharmacology, 2012, 64 (9), p. 1316-1325 (DOI: https://doi.org/10.1111/j.2042-7158.2012.01511.x)
- V. Saluja, J.P. Amorij, J.C. Kapteyn, A.H. de Boer, H.W. Frijlink, W.L.J. Hinrichs, A comparison between spray drying and spray freeze drying to produce an influenza subunit vaccine powder for inhalation, Journal of Controlled Release, 2010, 144 (2), p. 127-133 (DOI: https://doi.org/10.1016/j.jconrel.2010.02.025)
- E.M. Westerman, A.H. De Boer, P.P.H. Le Brun, D.J. Touw, A.C. Roldaan, H.W. Frijlink, et al., Dry powder inhalation of colistin in cystic fibrosis patients: A single dose pilot study, Journal of Cystic Fibrosis, 2007, 6 (4), p. 284–292 (DOI: https://doi.org/10.1016/S0939-6411(02)00044-9)
- E.M. Westerman, A.H. de Boer, P.P.H. Le Brun, et al., Dry powder inhalation of colistin sulphomethate in healthy volunteers: A pilot study, International Journal Pharmaceutics, 2007, 335 (1-2), p. 41–5
- A.H. de Boer, P. Hagedoorn, E.M. Westerman, P.P.H. Le Brun, H.G.M. Heijerman, H.W. Frijlink, Design and in vitro performance testing of multiple air classifier technology in a new disposable inhaler concept (Twincer®) for high powder doses, European Journal of Pharmaceutical Sciences, 2006, 28 (3), p. 171-178 (DOI: https://doi.org/10.1016/j.ejps.2005.11.013)
- P.P.H. Le Brun, A.H. de Boer, G.P.M. Mannes, D.M.I. de Fraîture, R.W. Brimicombe, D.J. Touw, A.A. Vinks, H.W. Frijlink, H.G.M. Heijerman, Dry powder inhalation of antibiotics in cystic fibrosis therapy: part 2: Inhalation of a novel colistin dry powder formulation: a feasibility study in healthy volunteers and patients, European Journal of Pharmaceutics and Biopharmaceutics, 2002, 54 (1), p. 25-32 (DOI: https://doi.org/10.1016/S0939-6411(02)00044-9)
- A.H. de Boer, P.P.H. Le Brun, H.G. van der Woude, P. Hagedoorn, H.G.M. Heijerman, H.W. Frijlink, Dry powder inhalation of antibiotics in cystic fibrosis therapy, part 1: development of a powder formulation with colistin sulfate for a special test inhaler with an air classifier as de-agglomeration principle, European Journal of Pharmaceutics and Biopharmaceutics, 2002, 54 (1), p. 17-24 (DOI: https://doi.org/10.1016/S0939-6411(02)00043-7)